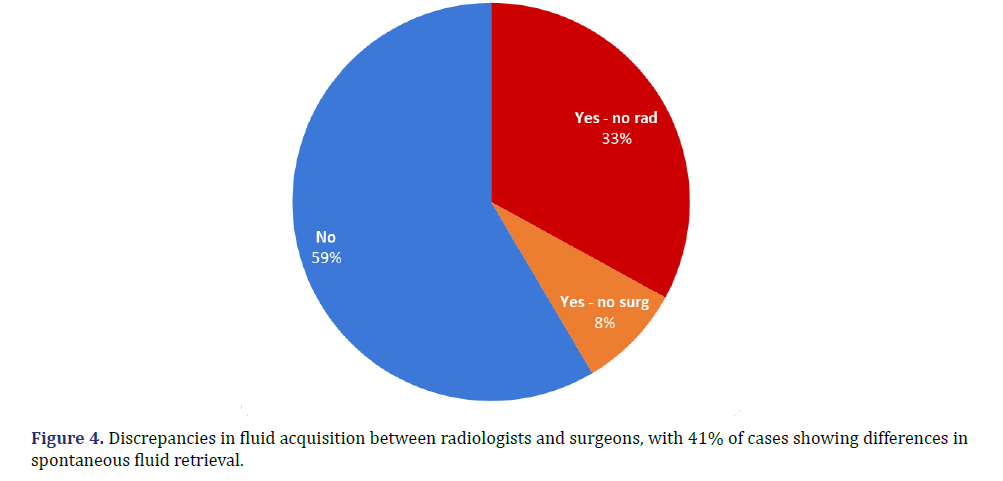Research Article - Journal of Contemporary Medical Education (2024)
Diagnostic Accuracy of Image-Guided Hip Joint Aspirations and Analysis of Predictive Parameters
Caleb Bhatnagar, Andrew Pasion, Elizabeth Sager, Mathew Illimoottil, Stanley Chu and Emad Allam*Emad Allam, Department of Radiology, Loyola University Medical Center, Illinois, USA, Email: Emad.allam@lumc.edu
Received: 03-Sep-2024, Manuscript No. JCMEDU-24-147268; Editor assigned: 05-Sep-2024, Pre QC No. JCMEDU-24-147268 (PQ); Reviewed: 19-Sep-2024, QC No. JCMEDU-24-147268; Revised: 26-Sep-2024, Manuscript No. JCMEDU-24-147268 (R); Published: 03-Oct-2024
Abstract
This study compares how well the findings from image-guided hip joint aspirations match those from subsequent surgery and investigates the factors that may explain any differences. We reviewed hip aspirations done from September 2014 to January 2023. Patients who had hip surgery within six months after aspiration were included, except those with incomplete records. We looked at differences in culture results and fluid samples between radiological and surgical procedures. Out of 94 hip aspirations, 63% were patients who had prior hip replacements. We found discrepancies in culture results in 17% of cases. In 69% of these, hip aspiration samples showed no bacterial growth, while surgery samples found growth. Delays in processing the samples correlated with these discrepancies (p=0.0166). P. acnes showed a significant tendency for discrepancies (p=0.0001). Differences in fluid acquisition occurred in 41% of cases, but lavage improved culture sensitivity by 80%. Overall, there are notable differences between image-guided hip aspirations and surgical findings. To improve results, lavage should be performed when there is no spontaneous aspiration of fluid, samples should be processed expeditiously, and special cultures should be used for fastidious organisms.
Keywords
Image-guided aspiration; Fluoroscopy; Ultrasound-guidance; Joint aspiration; Hip surgery; Aspirate culture
Introduction
Image-guided hip joint aspirations are routinely performed to exclude infection, with sensitivity and specificity reported around 64% to 89% in the literature [1-3]. Hip joint aspiration may be performed utilizing either fluoroscopy or ultrasound technology. For patients who are not obese, anatomic landmarks may be used to aspirate the joint. Sensitivity and specificity using this technique without imaging technology has been reported to be 78% and 93% respectively [4]. Fluoroscopy or ultrasound-guided aspirations are commonly performed in patients with a hip arthroplasty to exclude prosthetic joint infection. Analysis of synovial fluid typically includes red blood cell count, white blood cell count, polymorphonuclear cell percentage, and aerobic and anaerobic bacterial cultures [5]. Clinical data that may suggest infection/inflammation includes elevated levels of C-reactive protein and erythrocyte sedimentation rate [5]. Theoretically, percutaneous aspiration may underestimate infection due to: supine positioning resulting in posterior pooling of the fluid making it less accessible, complex viscous fluid which may not be amenable to aspiration, patient factors such as Body Mass Index (BMI) or pain making the procedure challenging, and pre-procedural antibiotics decreasing the culture positivity [6]. There may be variability in aspiration technique depending on the proceduralist which may also affect diagnostic accuracy. A common occurrence during hip aspirations includes a dry tap where no fluid is aspirated. In this scenario, a lavage with sterile saline may be performed with subsequent aspiration. Due to dilutional effects, cell counts are not recorded if lavage is performed and only cultures are grown.
Materials and Methods
We completed a comprehensive retrospective chart review of all patients who underwent hip aspirations between September 2014 and January 2023 at a large quaternary academic medical center. We included patients who had hip surgery within six months after the aspiration to allow for relevant comparison of aspiration with surgical results. Any patient with incomplete records was excluded from the study. All patients with successful fluid sampling by radiology and/or surgery were compared. Based on our inclusion and exclusion criteria, we collected data from 94 hip aspirations for statistical analysis.
The mean age of the patients was 56.6 years. 60% of the patients were male while 40% were female. The majority of hip aspirations (63%) were performed on patients with prior ipsilateral total hip arthroplasties to exclude prosthetic joint infection. For included patients, the collected data included patient demographics, medical history, details of the aspiration procedure, and surgical findings. For the aspiration procedure, we also recorded the method of aspiration visualization (fluoroscopy vs. ultrasound) to assess for any discrepancies.
Statistical analyses were employed to investigate the relationships between variables associated with hip aspirations and subsequent surgical outcomes. The chi-squared test of independence was used to assess associations between categorical variables, such as the presence of prior ipsilateral Total Hip Arthroplasty (THA) and discrepancies in fluid acquisition or culture results. For continuous variables, such as the time interval between aspiration and laboratory processing, a point-biserial correlation analysis was performed to examine the association with culture discrepancies. Additionally, chi-squared tests were applied to assess the relationship with specific microorganisms, such as P. acnes. All statistical tests were conducted with a significance threshold of 0.05.
Results
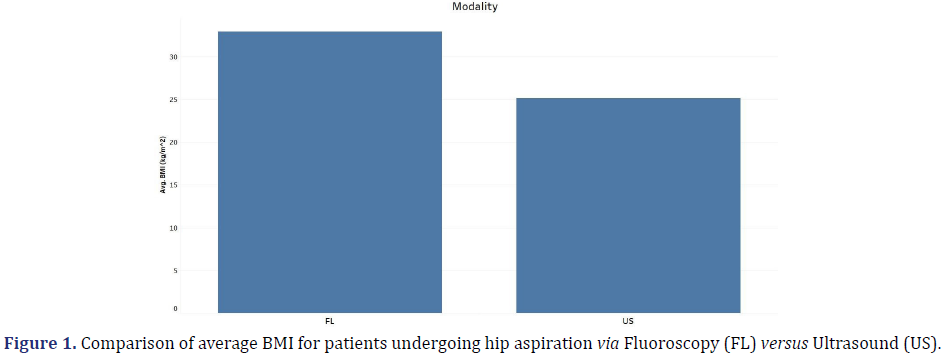
Of the 94 patients included in this study, the average BMI for patients undergoing hip aspiration via fluoroscopy was greater than that for aspiration via ultrasound. This is likely due to proceduralist preference as the hip may be harder to visualize with ultrasound in larger patients (Figure 1).
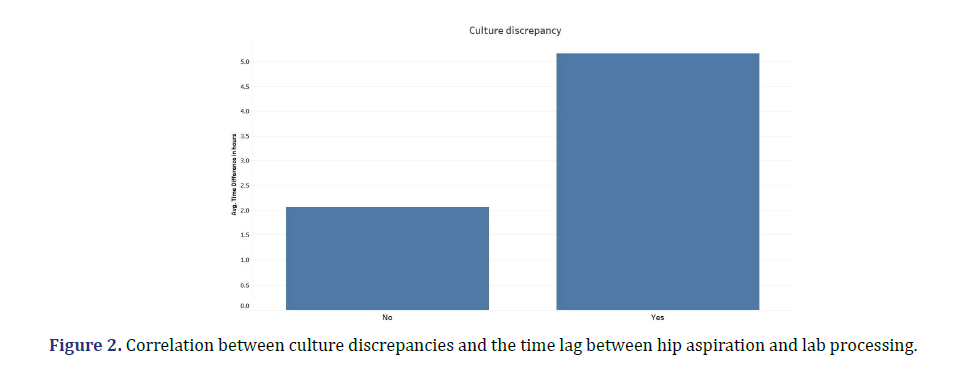
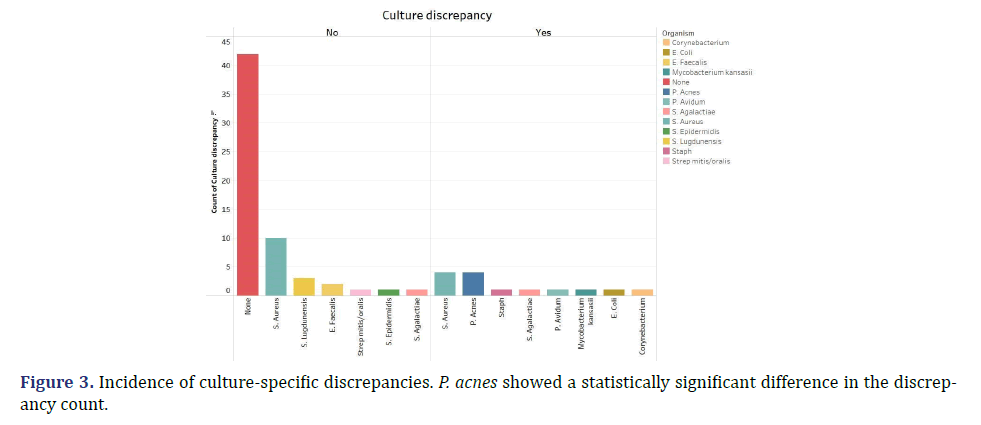
There was a discrepancy in bacterial growth recorded between cultures procured by the radiologist vs. the surgeon in 17% of cases. The majority of these (69%) were cases where there was no detectable growth in radiology samples, but growth of bacteria or mycobacterium in surgical cultures. There was a statistically significant correlation between culture discrepancy and the time lag between hip aspiration and lab processing (p-value= 0.0166). The greater this time interval, the greater the likelihood of discrepancy, with a threshold of two hours considered significant (Figure 2). Additionally, certain microorganisms showed a greater propensity for culture discrepancies. Notably, P. acnes growth was significantly more likely to be discrepant between hip aspiration and surgical procedures compared to other cultured organisms (p-value=0.0001). Every instance of P. acnes was characterized as discrepant i.e. detected by surgical cultures but not by radiology cultures (Figure 3). This may be attributed to its fastidious nature and potential evasion by standard detection methods and timelines.
There was a discrepancy in whether fluid was spontaneously obtained by the radiologist vs. the surgeon in 41% of cases. Specifically, in 33% of cases, the surgeon retrieved fluid from the affected hip at the time of surgery, whereas the radiologist had previously reported unsuccessful aspiration. Conversely, in 8% of cases, the radiologist was able to aspirate fluid from the hip, but this was not corroborated in the subsequent surgical report (Figure 4). Note that this excludes fluid obtained by the radiologist after lavage (injection of sterile saline).
In 22% of all cases, there was no spontaneous aspiration of fluid, prompting the radiologist to resort to lavage to attempt fluid collection. Among these, 19% culminated in positive cultures despite initial unsuccessful aspiration. In these cases, they were able to avert potential culture discrepancies in comparison to surgical reports. There was one instance of discrepancy despite lavage, in which there were both negative aspiration and lavage results; however, surgical findings showed positive P. acnes growth. This again points to the fastidious nature of P. acnes organism and the need for special consideration of this organism. Overall, in cases lacking initial spontaneous fluid aspiration under imaging guidance, lavage improved the sensitivity of cultures by 80%, identifying four out of five cultures that would have otherwise tested negative.
A comprehensive multivariate analysis revealed that other factors, such as age, gender, BMI, laterality, patient WBC count, presence of total hip arthroplasty, radiologist experience, fluoroscopy time, time between radiology procedure and surgery, antibiotic administration, and resident involvement did not result in statistically significant differences in discrepancy rates.
Discussion
In this analysis, we found clinically significant differences when comparing image-guided hip joint aspirations to subsequent surgical findings. Overall, there were discrepancies in 17% of culture results. There was a higher rate of false negatives in radiology cultures (69% of the discrepant cases) indicating that improved techniques are necessary for collecting and processing samples. Specifically, we found that delays in processing samples led to additional discrepancies. Processing samples within two hours reduced discrepancies [7]. This points to the importance of limiting delays following collection of fluid. This may require aid with transportation of samples and collaboration with the laboratory to expedite processing after aspiration. Instituting protocols to ensure prompt processing of samples after collection may improve the quality of results from hip aspiration procedures.
P. acnes was often missed in radiology cultures in comparison to surgical cultures. This organism has a tendency for slow growth and is difficult to detect with standard methods. This indicates that with current techniques, if there is a high clinical suspicion for infection, negative aspiration cultures may not be able to rule out P. acnes infection. This suggests the need to use specialized culture techniques for such organisms to reduce these misses [8,9]. For example, using an extended incubation period or taking additional samples for growth on various media may increase sensitivity. This again may require collaboration with the microbiology team for optimal sample processing with the goal of limiting cases of P. acnes that are missed on joint aspiration. Additionally, the development of more sophisticated culture techniques and rapid diagnostic tests for fastidious organisms could further improve the accuracy of diagnosing hip joint infections. Proprietary tests such as the Karius TestTM and SynovasureR may be considered in the appropriate clinical setting.
There was also a discrepancy in the ability of specialists to collect fluid on aspiration vs. surgery in 41% of cases. This includes cases where either the radiologist or surgeon was not able to obtain fluid from the hip. This might be due to differences in technique, anatomy, or timing. We found that using lavage improved culture sensitivity in cases where initial fluid aspiration failed. This suggests that lavage should be considered as the next step in cases where fluid cannot be aspirated initially. Lavage thus allows for adequate fluid to be obtained and improves microbial detection.
This study has several limitations as a retrospective analysis performed at a single center. It is subject to the inherent biases of retrospective studies, including selection bias, incomplete data records, and potential confounding variables. A prospective approach in which patients are enrolled at the time of initial hip joint aspiration would allow for further limitation of these biases. However, this retrospective analysis allowed for us to look at aspirations over a significant time frame of about nine years, expanding the number of data points available. The study was conducted at a single center, which may limit the generalizability of the findings. These discrepancies may be a result of the specific radiology and surgical procedures in place at our institution. Additionally, site-specific sample processing techniques may play a role. We also did not account for the potential skill level and ability of individual specialists to influence the level of discrepancy discovered in our analysis.
Our results lead to important considerations for reducing the discrepancies between joint fluid collection results. This fills a gap in current research, as this study works to provide tangible options to improve correlation of aspiration results. Our study takes a deeper look at reasons for discrepancies and practical points to help overcome those discrepancies beyond what is available in the current literature. However, further prospective, multicenter studies are warranted to validate our findings and refine broad recommendations for clinical practice [9]. Future studies are necessary to focus on standardizing protocols for image-guided aspirations, including the optimal technique, use of lavage, and expedited lab processing. Prospective studies comparing different imaging modalities (e.g. fluoroscopy vs. ultrasound) in guiding hip aspirations could also provide valuable insights into optimizing procedural techniques.
Conclusion
There are frequent differences between the results of image-guided hip aspirations and subsequent surgeries. In order to limit discrepancies and improve accuracy, we recommend using specialized cultures for fastidious organisms, specifically P. acnes. In addition, we recommend placing a high priority on processing the samples in a timely manner, limiting the time to initial processing to under two hours. Finally, we recommend utilizing lavage when fluid is not collected spontaneously to allow for greater detection of organisms when present. Following these recommendations may improve the accuracy of diagnosing hip joint infection by aspiration and reduce discrepancies between subsequent surgical results. This can improve the quality of joint aspiration procedures and hence improve patient care.
References
- Duck H, Tanner S, Zillmer D, Osmon D, Perry K. Value of ultrasound-guided aspiration of hip arthroplasties performed in an orthopedic clinic by orthopedic surgeons. J Bone Jt Infect 2021;6(9):393-403.
[Crossref] [Google Scholar] [Pubmed]
- Kanthawang T, Bodden J, Joseph GB, Vail T, Ward D, Patel R, et al. Diagnostic value of fluoroscopy-guided hip aspiration for periprosthetic joint infection. Skeletal Radiol 2021;50(11):2245-2254.
[Crossref] [Google Scholar] [Pubmed]
- Barker CJ, Marriot A, Khan M, Oswald T, Tingle SJ, Partington PF, et al. Hip aspiration culture: Analysing data from a single operator series investigating periprosthetic joint infection. J Bone Jt Infect 2021;6(6):165-170.
[Crossref] [Google Scholar] [Pubmed]
- Li R, Li X, Ni M, Zheng QY, Zhang GQ, Chen JY. Anatomic landmark-guided hip aspiration in the diagnosis of periprosthetic joint infection. Orthopedics 2021;44(1):e85-e90.
[Crossref] [Google Scholar] [Pubmed]
- Parvizi MD, Gehrke MD. Definition of periprosthetic joint infection. J Arthroplasty 2014;29(7):1331.
[Crossref] [Google Scholar] [Pubmed]
- Chiodo CP, Logan C, Blauwet CA. Aspiration and injection techniques of the lower extremity. J Am Acad Orthop Surg 2018;26(15):e313-e320.
[Crossref] [Google Scholar] [Pubmed]
- Barrack RL, Harris WH. The value of aspiration of the hip joint before revision total hip arthroplasty. J Bone Joint Surg Am 1993;75(1):66-76.
[Crossref] [Google Scholar] [Pubmed]
- Li R, Lu Q, Chai W, Hao LB, Lu SB, Chen JY. Saline solution lavage and reaspiration for culture with a blood culture system is a feasible method for diagnosing periprosthetic joint infection in patients with insufficient synovial fluid. J Bone Joint Surg Am 2019;101(11):1004-1009.
[Crossref] [Google Scholar] [Pubmed]
- Spangehl MJ, Masri BA, O'Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am 1999;81(5):672-683.
[Crossref] [Google Scholar] [Pubmed]